#Dot Gain Control in Hot Melt Inkjet Inks
Hot melt inkjet printing has become a go-to solution for high-resolution, high-speed digital printing across industries. Its ability to produce sharp images with quick drying times makes it invaluable, but achieving consistent print quality isn’t without its hurdles. A well-engineered ink is crucial to producing high quality images with vibrant colors and crisp edges.
A key factor in achieving this level of performance is dot gain, which refers to the spread of an ink droplet on a substrate. For chemists and formulators working with hot melt inkjet inks, precision control over dot gain is paramount. This article explores the challenges associated with controlling dot gain and demonstrates how one can overcome the limitations of conventional waxes used in hot melt inks.
#The Role of Dot Gain in Achieving High-Resolution Digital Prints
Hot melt inkjet inks typically are composed of waxes, resins, colorants, and other additives formulated to impart the properties needed for proper jetting and image quality. Dot gain is a major driver of image quality, but can be difficult to control while maintaining jetting performance.

#Array of ink drops deposited on a substrate, initial state.
For successful droplet formation, inkjet fluids must have a relatively low viscosity (<20 mPa-s) during jetting. The deposited fluid initially forms an array of discrete drops on the substrate. Left on its own, the fluid will begin to flow and spread. Ideally, the droplets will coalesce into an even layer before they are pinned in place as they cool and freeze. If the droplets flow too much, they can spread beyond their deposition area, causing the image to lose resolution and blurring edges.
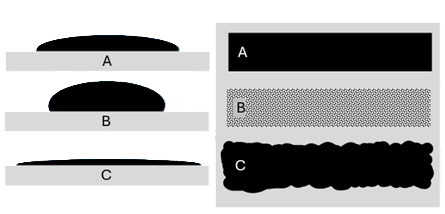
#Deposited drops on a substrate. A-Optimal drop spread; B- minimal dot spread results in "white space" and low optical density; C- excessive dot spread, loss of edge definition
Dot gain refers to the degree of this droplet spread, coalescence, and wetting before they are pinned to the substrate. If the dot gain is too small, droplets will fail to coalesce into a level film and produce poor color quality. If dot gain is too large, the droplets will flow beyond their deposition area and produce blurry images. Controlling dot gain properly can be the difference between poor image quality and stunning, vibrant graphics.
#Key Factors Impacting Dot Gain in Hot Melt Inkjet Ink Formulations
Dot gain is the result of a complex interaction between fluid viscosity, contact angle, substrate wetting, and the amount of time the fluid has to flow on the substrate before being pinned in place, also known as the open time. The fluid viscosity, contact angle, and wetting are to some extent immutable due to the requirements for jetting on a given substrate, but the open time is not.
In UV and solvent-based inks, open time is relatively straightforward to control through the use of UV and drying lamps. In contrast, hot melt inks are more difficult to control because the open time is determined by the melt/freeze behavior of the wax used in the ink formulation.
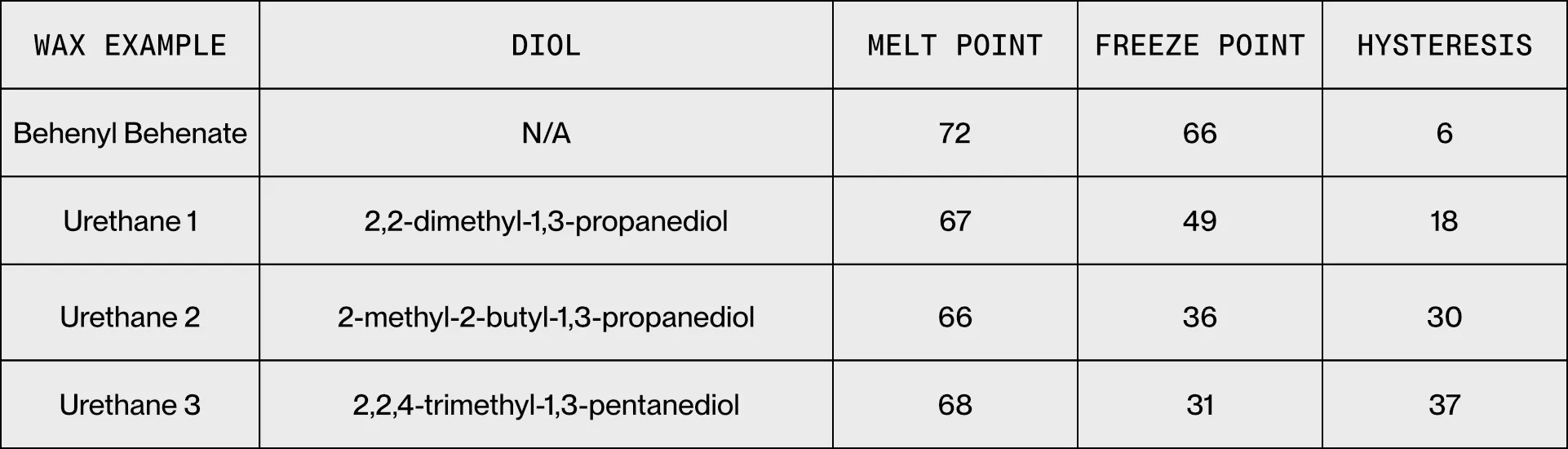
Commercially available waxes used in hot melt inks are often comprised of one or more long-chain hydrocarbon groups connected via ester or amide linkages. These materials have moderately broad phase change transitions with little difference between the melt and freeze points. This difference is known as the phase change hysteresis. For example, behenyl behenate is a commonly used wax in hot melt inks. It has a small phase change hysteresis, with the differential scanning calorimetry (DSC) profile showing that minimal the melt and freezing transitions have minimal offset and even has some overlap in temperature.
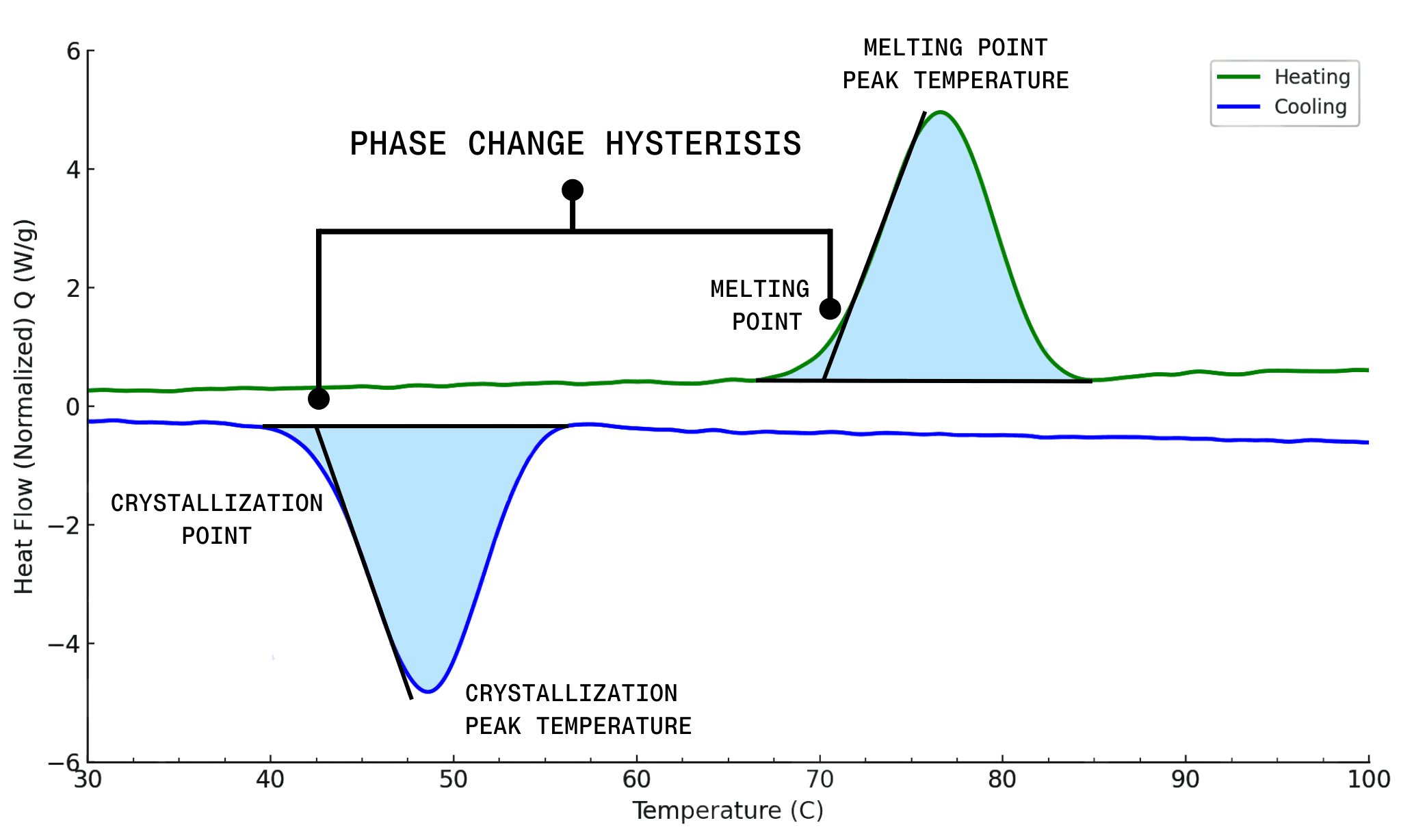
#DSC Phase Change Hysteresis
In practice, an ink with a small melt hysteresis will rapidly solidify after being deposited on a substrate, thus having a small open time and therefore low dot gain. One can solve this by allowing the ink to remain in liquid state long enough to get closer to the ideal level film. This melt hysteresis behavior can be precisely tuned with a properly designed wax.
#How Custom Wax Chemistry Enables Precise Dot Gain Control
Crystallization in hydrocarbon waxes is primarily governed by van der Waals forces, but incorporation of other chemical functionality into the wax molecule can introduce dipole-dipole and even hydrogen bonding forces to control both primary and secondary crystallization. The crystallization kinetics can be further moderated by incorporating asymmetrical, sterically bulky groups between the long-chain hydrocarbons. By controlling both the crystallization point and kinetics, one can design a wax with a tailored phase change hysteresis.
This is shown by Woudenberg and Secord in US 9,944,806 where sterically bulky aliphatic diols are reacted with stearyl isocyanate to produce urethane waxes with phase change hysteresis. Three examples are listed with behenyl behenate as a reference. Following this approach, they were able to program the melt hysteresis between 6 C and 37 C, while leaving the melting point largely unchanged. This toolbox allows for fine control of dot gain, thus enabling formulation of printing inks with optimized dot gain and excellent image quality.
#Structures of synthesized waxes
#Scale Up Custom Wax Synthesis for Inkjet Ink Formulations with DigiChem
Custom waxes are a powerful tool for formulating high performance hot melt printing inks. DigiChem makes it easy to develop and commercialize these materials through our intuitive, online process.
- Draw your molecule in our online tool or upload a chemical structure
- Set your quantity and purity specifications
- Get a quote and place your order online
- Receive your material quickly, and work on your ink formulations
When you need to scale to pilot production, we are here for you. DigiChem gives you access to a network of toll manufacturers and suggests ideal partners based on your needs. We are here to ensure that you get to market as quickly and efficiently as possible.
Start building your custom wax now using the DigiChem molecule builder: chemos.digichem.com - engineered for formulators who need results, fast.
References